VAPCare Intelligent Device Helps Both Medical Staff and Patients to Fight Coronavirus Pandemic
Introduction
According to the CDC, Ventilator-associated pneumonia (VAP) is a lung infection that develops in a person who is on a ventilator. An infection may occur if germs enter through the tube and get into the patient’s lungs.
VAP is the most common lethal infection observed in patients who require treatment in intensive care units (ICUs). It’s defined as pneumonia occurring at least 48 h after intubation and the start of mechanical ventilation. This time window is important so that any infection that is incubating at the time of admission can be excluded.
VAP is one of the most common and most critical hospital-acquired infections with extremely high mortality rates (42%). VAP is quite common among patients in critical care settings.
Close to 50 million patients across the world enter intensive care units (ICUs) annually, and 19 million patients get on long-term ventilation (over 48 hours), while 31% of them develop VAP during their stay in the ICU. Not only does it lead to 2.5 million deaths annually, it puts stress on ICUs and also increases the cost of treatment.
Unfortunately, the consistency of secretion management and oral hygiene protocols is often poor. In fact, this is a leading cause of VAP, resulting in 6,00,000 cases in India every year.
VAPCare – A Life Saving Device
Now, The Bengaluru-based startup Coeo Labs, the critical care division of InnAccel Technologies. India’s first product innovation platform with a diverse portfolio of globally patented medical technologies has launched VAPCare, a device that helps reduce deaths due to infections that pneumonia patients who are on a ventilator develop.
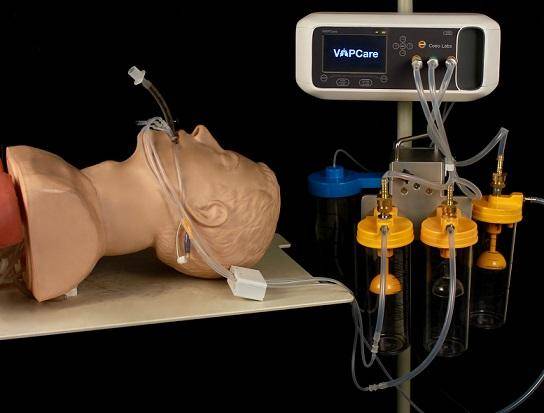
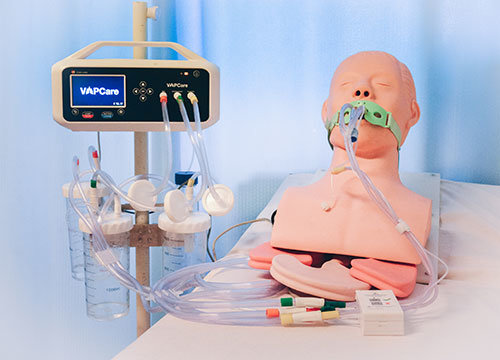
VAPCare is an intelligent, secretion management, and oral hygiene system for ventilated patients in the ICU to reduce the chances of aspiration of the contaminated secretions. It’s a patented AI-based comprehensive and intelligent secretion management and oral hygiene system for ventilated patients in the ICU. This system eliminates the need for constant human interaction between caregiver and patient to prevent the chances of cross-infection.
Coronavirus causes a respiratory disease that can spread to other people when they come in contact with the respiratory droplets of the affected person. Healthcare providers who need to suction saliva and secretions from ventilated coronavirus patients are at risk of contracting the virus.
Usually, the removal of secreted fluids has to be done manually by a nurse and is time-consuming. This device becomes an important life saving for many medical professionals as they have direct contact with coronavirus patients and this virus is very contagious.
According to company website, the key technology features are:
• Automated secretion clearance from Oral, Oropharyngeal and Subglottic (through ET tube with Subglottic port) regions
• Automated oral lavage User-defined suction frequency and pressure
• Sensor-controlled suction duration
• Automated port block detection and management
• Compatible with standard ET, CASS/SSD, and tracheostomy SSD tubes
Coeo Labs’ product VAPCare reduces chances of acquiring VAP by following key functions:
• Artificial Intelligence and sensor-based secretion management from 3 locations: Oral, Oropharyngeal, and Subglottic regions.
• Independent suction pressure control in each line
• Automatic detection and management of port block
• Automatic regular mouthwash and lavage
• Compatible with all ET Tubes
A Vijayarajan, CTO, InnAccel Technologies said, “We wanted to address this problem with VAPCare, which automatically removes saliva before it reaches the lungs and also pushes the anti-microbial liquid into oral cavities. It increases ICU efficiency and eliminations multiple risk factors that are associated with the manual-suctioning process.”
VAPCare has undergone extensive technical and clinical evaluation. A 30-patient clinical evaluation was recently completed with excellent results. The device not only improved patient safety by bringing down cases of mucosal injury but also increased the effectiveness of secretion clearance. Most importantly, no incidents of VAP were reported.
VAPCare was developed with support from the Biotechnology Industry Research Assistance Council, an Indian government body that provides funding and grants for research. The device has also been approved by the U.S. FDA (Food and Drugs Administration) and has received many innovation awards in India and abroad.